Nanodiamond is the term used to describe diamond particles so small that they are measured in nanometers, or billionths of a meter. Nanodiamonds are very, very small and diamond is pure carbon in its hardest state.
These tiny diamonds can be created through several methods including the explosive detonation of a carbon producing explosive. Their size typically ranges from 3-5 nm to 100 nm.
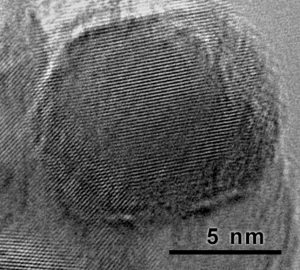
Explosive nanodiamond synthesis is achieved in one of two ways. One method involves adding graphite to an explosives system and subjecting the graphite (carbon) to the shock wave of an explosive detonation. The other method is to detonate a carbon producing explosive inside of a thick pipe. In both cases, a portion of the carbon is converted into diamond and some remains as graphite, which can be burned off with strong acids. Diamond material created from the detonation of an explosive is referred to as “detonation nanodiamond”.
Nanodiamond can also be synthesized from a suspension of graphite in an organic liquid at atmospheric pressure and room temperature using ultrasonic cavitation. Another alternative method is Chemical Vapour Deposition, a technique that involves depositing diamond slowly on a material through a process that makes use of a filament, gaseous carbon source, and microwave energy. These methods can produce small amounts of high purity nanodiamond.
Nanodiamond is typically used in specialty coatings, lapping and polishing applications, and as an additive in polymer processing. Nanodiamond material has widely been used as an additive to lubricating oils to reduce friction between moving parts to increase utility. New applications are for data storage and the delivery of drugs to the body.
Nanodiamond material can be mixed with organic or metallic binding materials to produce what the industrial diamond industry refers to as resin-bond diamond or metal-bond diamond, which is used to produce cutting tools in the abrasives industry.
References:
1. Dolmatov, V. Y., Detonation synthesis ultradispersed diamonds: properties and applications, Russian Chemical Reviews, 70, 607, 2001.
2. Shenderova, O.A., Zhirnov, V.V., and Brenner, D.W., Carbon nanostructures., Critical Rev. in Solid State and Materials Sciences, 27, 227, 2002.Link
3. Detonation Nanodiamonds and Related Materials, Bibliography Index, First Issue, Eds. A. Vul, V. Dolmatov and O. Shenderova, “FIZINTEL”, S.Petersburg, Russia, 2003.
4. Ultrananocrystalline Diamond: Synthesis, properties and Applications, edited by D.Gruen, A.Vul and O.Shenderova, NATO Science Series, Kluwer Acad.Publ., 2005.